Egg Science: Bouncing Egg Experiment
March 2024 | Molly S.
Engage students with an exciting chemistry lesson that connects class chickens with laboratory science. Egg membranes can be difficult to see, even when viewing a chicken egg raw vs. hardboiled. This experiment use common household items to magically disintegrate the hard, calcium carbonate eggshell to reveal an elastic membrane of an egg.
Key Concepts: Compounds, Acids, Salts, Anions, Cations
Time Estimate:
10 minutes (execution & explanation) 24 hours (wait time)
Activity Outline:
Turn an ordinary chicken egg into a bouncy ball using vinegar!
Leave a regular chicken egg in a bottle of vinegar overnight. The eggshell will disintegrate, leaving behind an elastic membrane.
Materials List:
- White Vinegar
- Egg
- Glass container taller than egg
- Something to cover the top of the container.
- Gloves
- Safety glasses
- Expo marker or Sharpie
Science Background
While the egg is sitting in vinegar, what is happening?
Eggshells are made of calcium carbonate (CaCO3), a chemical compound often found in marine animals, rocks, and snail shells. The calcium is what makes the eggshell hard to protect the embryo, or baby chick inside.
Vinegar is an acid. This is because it contains a compound called acetic acid (CH3COOH). When the acid comes into contact with the calcium carbonate, a salt, the eggshell will start to break down.
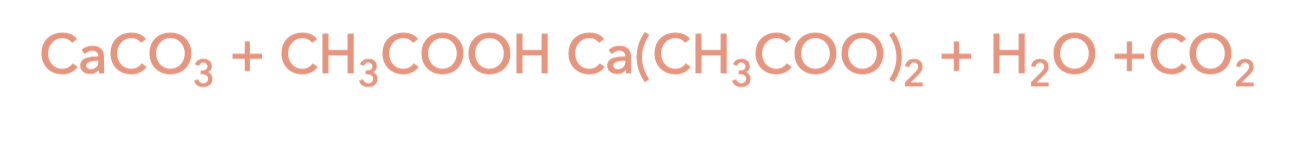
A salt is a compound made of a substance with a positive charge (cation) and a substance with a negative charge (anion). In this case, the calcium (Ca) is a cation, and the carbonate (CO3) is the anion. The calcium carbonate reacts with the acetic acid to create calcium acetate (Ca(CH3COO)2 ), water (H2O), and carbon dioxide (CO2). The carbon dioxide creates the bubbles you see on the outside of the eggshell in the experiment!
When the acetic acid comes into contact with the shell, the calcium and carbonate break apart causing the eggshell to disappear, leaving behind the flexible membrane on the inside of the shell which protects the albumin (egg protein) from the vinegar. Now your egg bounces!
Instructor Preparation:
Engage:
- What happens when you drop a chicken egg?
- Have you ever wondered why eggshells are so fragile?
- Have you ever seen an egg bounce?
- Why do chickens have eggshells?
Explore:
- Label container and ensure class is wearing PPE.
- Ask students questions above. What are their hypotheses about what will happen?
- Place egg into the container.
- Pour white vinegar into the container until it covers the egg.
- Cover the container (it will smell) and let stand for 24 hours.
- Have kids write down initial observations (bubbles, smell, look, etc.) and if possible, have them observe again every couple of hours and write down observations.
- After 24 hours, note final observations and gently remove the egg from the container. Rinse (gently) with water, then bounce from a couple inches off the table!
Explain:
- Ask questions to help kids reflect on their exploration.
- Why do you think there were bubbles on the outside of the egg? Why do you think the egg needs to sit in vinegar for so long?
- If the experiment did not work, why? What can we change if we were to do this experiment again?
It may be helpful to watch the video demonstration of this lesson with your class to explain the process visually.
Elaborate:
- How high can you bounce the egg before it breaks? Can you bounce it from higher if it lands on a soft surface?
- What are other ways you can protect an egg? This activity can be extended by creating capsules to protect an egg as it drops from a certain height.


